Which of the Following Has the Highest Ionization Energy
Ionization energy of Seaborgium Sg 107. C r 1s22s22p63s23p63d54s1.
3 3 Trends In Ionization Energy Chemistry Libretexts
This gives the sensing chamber an effective electrical conductance.
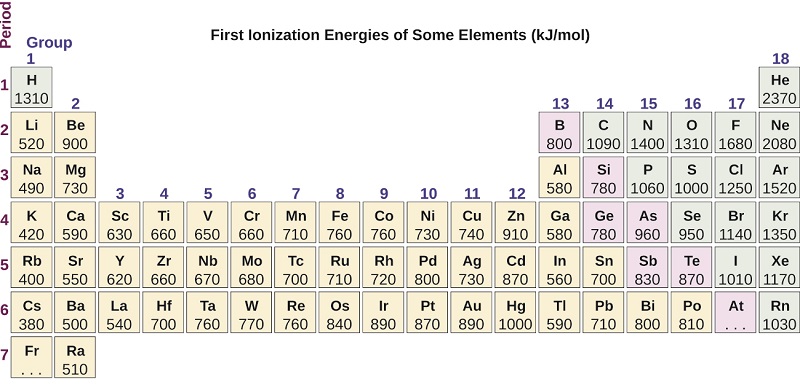
. The tabular chart on the right is arranged by Ionization energy. So the second ionization energy of sodium will be the highest as that would require extraction of an octet electronic configuration. The first ionization energy refers to the energy it takes to remove one electron from an atom.
See the answer See the answer done loading. Best Answer a Cl. From this trend Cesium is said to have the lowest.
Neon has the highest ionization energy. Ionization energy of Hassium Hs 109. Electronic configuration for chromium is.
119 rows - Ionization energy. Further the ionization energy of carbon is 108645 Kj m o l. The elements P Na and Cl belong to the third period.
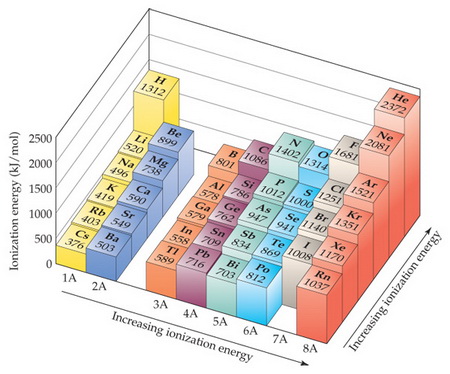
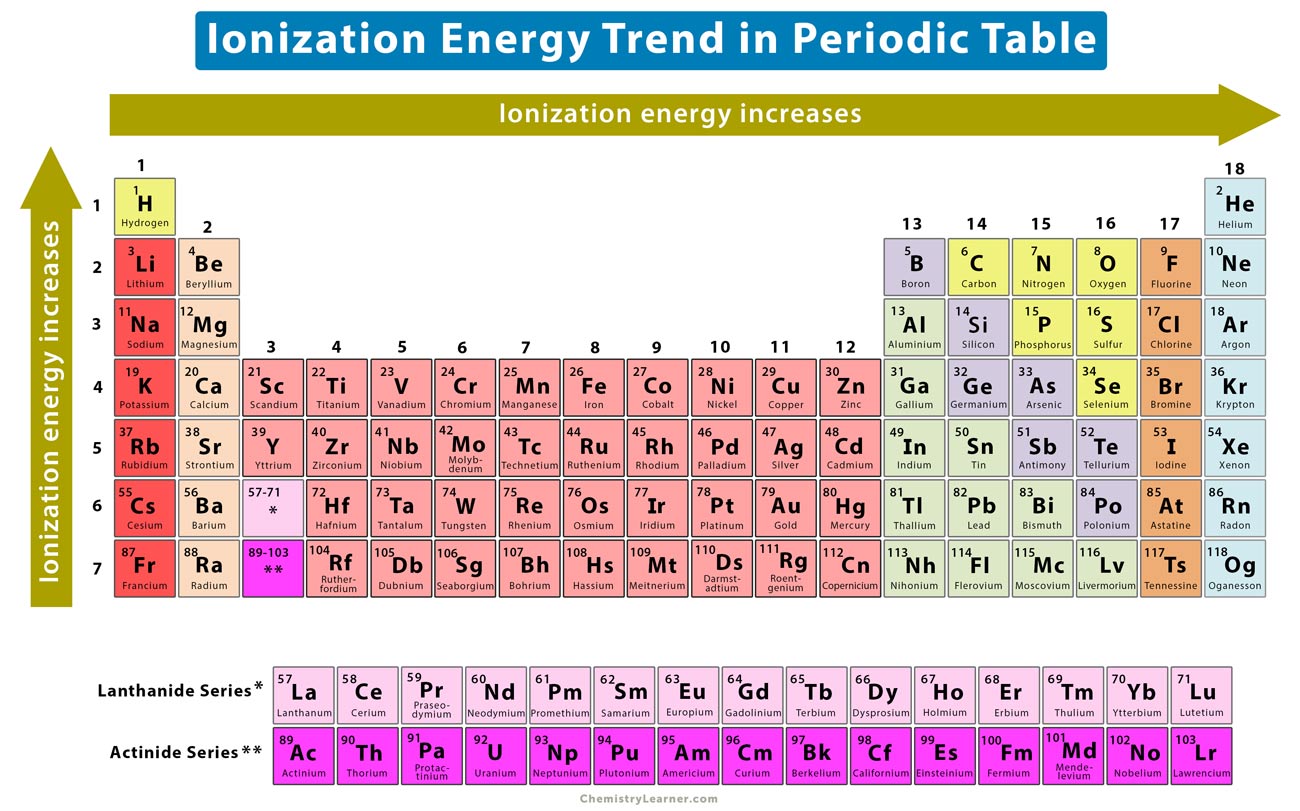
The first chemical element is Cesium and the last one is Helium. Ionization energy of Darmstadtium Ds 111. -Ionization energy increases from left to right across a row on the periodic table and from bottom to top in a column.
Hence the correct option is C. Alkali metals have very low ionization energies because they have only one valence electron to dose before attaining noble gas configuration. Of the following which element has the highest first ionization energy.
So carbon has the highest first ionization energy. Metals have low ionisation energy and non-metals have high ionisation energy. Order the elements Si S and F in terms of increasing ionization energy.
It mainly depends on the electrostatic attraction between the positive protons in the nucleus and negative electronsif protons and electrons are more attracted to each other then the first ionization energy would be higher and vice versa. List the following from lowest to bartleby. Inert gases have zero electron affinity because of their stable electronic configuration.
Check Answer and Solutio. A P Na Cl b F O Ne c Ne He Ar. List the following from lowest to highest first ionization energy.
Which element from the following has the highest ionization energy. Since the nuclear charge is necessarily diminished with respect to the valence shell the alkali metals display the lowest ionization energies. And across the period ionisation energy tends to increase.
Mg2 has highest ionization energy because its Ne electronic configuration which has smallest size among other two. Which has lowest first ionization energy. Thus its outermost orbit is completely occupied as it forms an octet and hence Neon is.
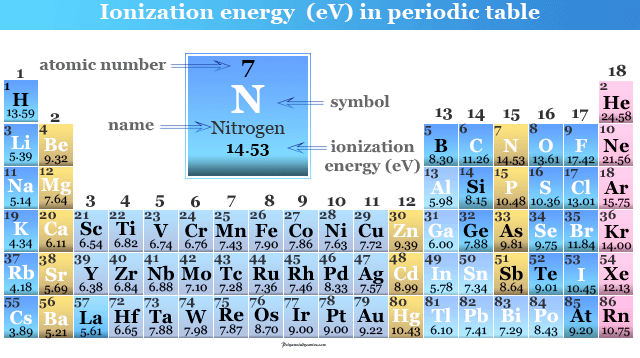
Which element has the highest ionization energy. Ionization energy of Meitnerium Mt 110. The unity for ionization energy is eV.
After ionisation of 4s electron the next electron which will be ionised is from 3d orbital. For chemistry students and teachers. When smoke particles enter the ionization area they decrease the conductance of the air.
120 rows Ionization energy of Rutherfordium Rf 6 eV 105. As 3d5 electronic configuration is stable hence the second ionisation of C r will require much greater ionisation energy than the expected one. The order of the ionization energies is Li.
Also across the period ionisation energy tends to increase. As there are two electrons in the valence shell of magnesium so the first ionization energy should be high. Video Explanation Solve any question of Classification Of Elements And Periodicity In Properties with-.
Hence option D is correct. Carbon has the highest first ionization energy. Generally metals have low ionisation energy and non-metals have high ionisation energy.
On moving from left t right in a period the ionization energy increases as the effective nuclear charge increases and the size decreases. Which has the higher ionization energy Na Mg2 or Cl-. Na - Group 1 P - Group 15 and Cl - Group 17.
And hence it has the highest ionization energy. 51 Which of the following elements would be expected to have the highest first ionization energy2 How many atoms of carbon are there in 20 moles of methane CH4. 11 rows The elements that belong to the noble gases or inert gases or Group VIII-A have the highest.
Ionisation energy of noble gas - Very high and decreases down the group - wherein. Going down the group ionization energy decreases and moving across a period ionization energy increases so scandium has the highest ionization energy. Among the following the third ionization energy is highest for A magnesium B boron C beryllium D aluminium.
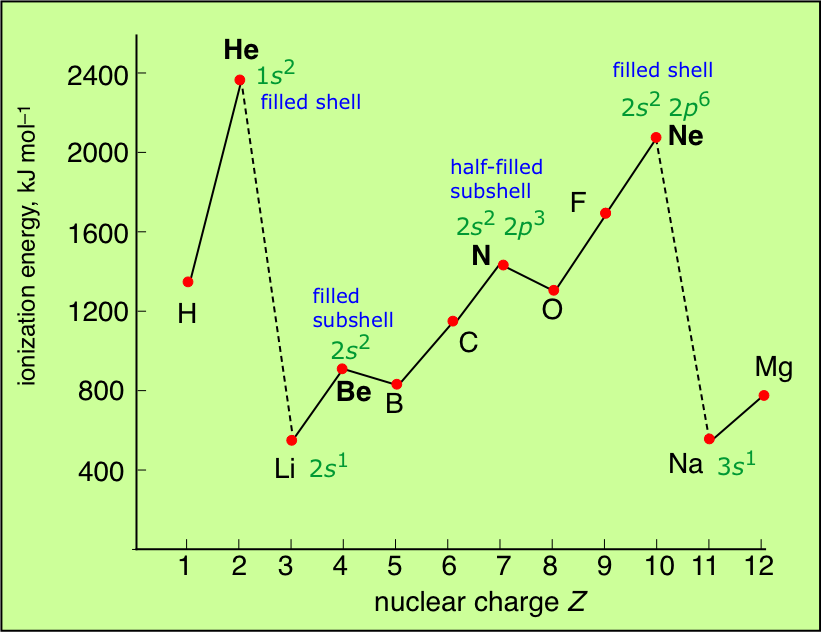
Thus helium has the largest first ionization energy. IE is inversely proportional to size. Electronic configuration can be written as 28.
This problem has been solved. So scandium is the only element that is not in the first group and has the highest IE among all. The ionization energy decreases from top to bottom in groups and increases from left to right across a period.
Which of the following has the highest ionization energy. Ionization energy of Bohrium Bh 108. Si S F Si S F are in order.
51 Which of the following elements would be expected to have the highest first ionization energy2 How many atoms of carbon are there in 20 moles of methane CH4. Mg - The electronic configuration Mg of is - 1 s 2 2 s 2 2 p 6 3 s 2. It has an atomic number of 10 with 8 electrons in its outermost orbit.
Group of answer choices. Ionization energy of Dubnium Db 106. C C has the highest ionization energy b.
An ionization smoke detector contains a material which ionizes the air in the sensing chamber thus rendering it conductive and permitting a current to flow between two charged electrodes. P Na and Cl are elements belonging to the.
Ionization Energy Definition Equation Periodic Table Trends

Inorganic Chemistry How Can I Relate The Reactivity Series To Electronegativity And Ionization Energy Chemistry Stack Exchange
How Would You Arrange The Following Elements In Order Of Increasing Ionization Energy Te Pb Cl S Sn Socratic

Do Nonmetals Have High Ionization Energy

Which One Has The Lowest First Ionization Energy Ca P Or Ci Quora
Ionization Energy And Electron Affinity

Why Is The Ionisation Enthalpy Of Tl Greater Than That Of In Quora

The Parts Of The Periodic Table
Ionization Energy Definition Chart Periodic Table Trend
Why Does Magnesium Have A Higher Ionisation Energy Than Strontium Quora

The Parts Of The Periodic Table
What Are The Periodic Trends For Atomic Radii Ionization Energy And Electron Affinity Socratic

Why Does Nitrogen Have A Higher Ionization Energy Than Carbon Quora
Highest Ionization Energy Quiz Questions Proprofs Quiz

Periodic Trends In Ionization Energy Ck 12 Foundation

Q Solved Which Element Requires The Least Amount Of Energy To Remove The Most Loosely Held Electron From A Gaseous Atom In The Ground State
Which Element Has The Highest First Ionization Energy Socratic
Comments
Post a Comment